Content
Penicillin

Blunting wonder weapon

Sir Alexander Fleming
Looking back, September 28, 1928 might have been one of the most important dates in the history of medicine. On this day, the Scottish doctor Alexander Fleming observed that a mould can kill bacteria. The method of action of penicillin was discovered.
Fleming (1881-1955) - by the way, he also discovered lysozome with its antibacterial properties - researched staphylococci at the St. Mary´s Hospital in London and had settled the bacteria on agar plates. After some time, mould had formed in the Petri dishes. Fleming noticed that there were no more bacteria in the vicinity of the fungus. He concluded that the fungus "Penicilium notatum" could kill certain bacteria. He called the agent “Penicillin”. Fleming wrote an article about his observations for the “British Journal of Experimental Pathology”, which appeared in 1929. Then, nothing happened.
Forgotten pioneers

Ernest Duchesne, an (almost) forgotten pioneer of Penicillium research
So Fleming could have done exactly like his predecessors: as early as 1870, the London physiology professor John Scott Burdon-Sanderson saw a connection between moulds and bacterial growth. In 1874, the famous Viennese surgeon Theodor Billroth is said to have recognised the bactericidal effect of Penicillium. The no less well-known British physician and antisepsis pioneer Joseph Lister also undertook healing experiments with Penicillium in Edinburgh in 1884. In Rome in 1893, Bartolomeo Gosio extracted the agent mycophenolic acid from a Penicillium species, which is regarded as the first antibiotic in history but fell into oblivion.
And in 1897 the young French military physician Ernest Duchesne (1874-1912) submitted his doctoral thesis, which described that certain molds are antibiotically effective. Duchesne had come to this conclusion because he had observed that stablemen deliberately stimulated the development of mould on saddles by storing them in dark, damp rooms. They told him that this would make the scratches on the horses' back heal faster. Duchesne gained a solution from the mould cultures and carried out successful animal experiments. But the Pasteur Institute rejected his dissertation. How different the history of medicine could developed!
The first antimicrobial drug was arsphenamine, introduced by Paul Ehrlich in 1910, which was used for Syphillis therapy under the trade name Salvarsan.
War accelerates development and mass production

Dr. Ernst Boris Chain
However, all these observations and research were not turning points in the history of medicine because they were not perceived or pursued by science - and no patents were applied for. Even after Alexander Fleming's publication on the Penicillium, not much happened for almost ten years, because the substance proved to be unstable and difficult to produce in its pure form.
However, in 1940 Ernst Boris Chain (a Berlin chemist who, like many other great German scholars, had fled to England to escape the Nazis), Howard Florey (an Australian pathologist) and their colleagues in Oxford succeeded in systematically producing a pure form of penicillin and investigating its qualities more closely. The Second World War multiplied the Allies' interest in effective remedies for inflamed wounds. Especially since the enemies in Germany had the Prontosil developed by Gerhard Domagk, the first real antibiotic on the market (penicillin, however, was superior to this drug, as should be proven).
In 1941, Florey and Chain undertook the first test on a human, which proved the effectiveness of penicillin, but ended tragically because the drug was not yet available in sufficient quantities.
Combined efforts on both sides of the Atlantic eventually led to an effective drug that could be produced in large quantities. In 1945 Fleming, Florey and Chain were awarded the Nobel Prize for Medicine. The triumph of penicillin began. It was produced in large quantities, spread all over the world and still saves the lives of countless people today.
Fleming early warned of the risks of bacterial resistance

Penicillin advertising in a pharmacy window in Germany, 1954
In the meantime, however, the problems caused by the mass use of antibiotics in recent decades have become the focus of attention: Many bacteria have become resistant.
Alexander Fleming had already pointed out this danger in his speech at the Nobel Prize ceremony in 1945: “It is not difficult to make microbes resistant to penicillin in the laboratory by exposing them to concentrations not sufficient to kill them, and the same thing has occasionally happened in the body.
The time may come when penicillin can be bought by anyone in the shops. Then there is the danger that the ignorant man may easily underdose himself and by exposing his microbes to non-lethal quantities of the drug make them resistant.”
The decades-long mass use of antibiotics in mass livestock farming and fattening, which end up in the environment, is particularly problematic. To this day, animals still are fed larger quantities of antibiotics than humans.
The development of resistances follows the classical Darwinian evolutionary concept: resistant bacteria are better able to reproduce because the non-resistant bacteria are killed and make room for them, and pass on their resistances in rapid succession to new generations, which in turn adapt better and better. This microevolution made species such as Staphylococcus aureus multiresistant.
More and more multi- and cross-resistant germs
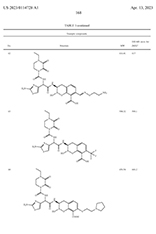
Detail from US020230114728A1: "Penicillin-binding protein inhibitors"
Medicine is thus facing an increasingly threatening problem. Quite a few patent applications in recent years - as a glance at the DPMA patent database DEPATISnet shows - are therefore devoted to the discovery or removal of antibiotics in animal feed.
Resistance detection is also an important topic, see for example ![]() WO002015164225A1 (2,53 MB) ("Rapid determination of bacterial susceptibility to an antibiotic at the point of supply").
WO002015164225A1 (2,53 MB) ("Rapid determination of bacterial susceptibility to an antibiotic at the point of supply").
Approaches such as ![]() EP3330275A1 (3,14 MB) ("Novel aminoglycoside antibiotic effective against multidrug-resistant bacteria") or
EP3330275A1 (3,14 MB) ("Novel aminoglycoside antibiotic effective against multidrug-resistant bacteria") or ![]() WO002018091707A1 (1,76 MB) ("New antimicrobial agents against Staphylococcus Aureus") give cause for hope.
WO002018091707A1 (1,76 MB) ("New antimicrobial agents against Staphylococcus Aureus") give cause for hope.
Recent filings include, for example, "penicillin-binding protein inhibitors" ( ![]() US020230114728A1 (9,27 MB)) or "penicillin-G acylases" (
US020230114728A1 (9,27 MB)) or "penicillin-G acylases" ( ![]() US020230272363A1 (6,42 MB)).
US020230272363A1 (6,42 MB)).
Despite all this, the potential of antibiotics is far from exhausted - see, for example, ![]() EP4108244A1 (2,7 MB), "Lactam antibiotic with significant activity against cancer."
EP4108244A1 (2,7 MB), "Lactam antibiotic with significant activity against cancer."
Pictures: iStock.com/catoll, via Wikimedia Commons, Wikimedia Commons, Nobel Prize Foundation, Bundesarchiv, Bild 183-23912-0002, DEPATISnet
Last updated: 8 January 2025

Not only protecting innovations
Social Media